Cold day ahead! High temp of 273..... WHAT?!
273 degrees! Water boils at 212 degrees, that's not cold at all?! I’m talking 273 KELVIN, which is the same as 0° Celsius and 32° Fahrenheit.
Whoah. Now you may be confused again. Are there really three scales to measure temperature? Yep, there is! In a previous post, I explained the difference between the Fahrenheit and Celsius scales. You can read that blog post by clicking HERE. Now, I’m going to dive into the Kelvin scale. You will never see kelvin temperatures on a weather map, except in the video forecast below.... However, the kelvin is an essential variable behind weather models.
The Celsius and Fahrenheit scales are based on the freezing and boiling point of water. The freezing point of water is 0° C or 32° F, and the boiling point of water is 100° C or 212° F. The Kelvin scale uses a new concept, absolute zero, which is 0 K.
As discussed in a previous post, the temperature is the energy of particles due to their movement. The slower the particles move, the lower the temperatures. Faster movement equates to higher temperatures. With the Fahrenheit and Celsius scale, we go into sub-zero temperatures. Particles are moving very slowly. Absolute zero describes the moment where the particles do now move at all. At this point, the temperature cannot go any lower. This happens at 0 K, which equates to -273° C. There are no negative temperatures on the Kelvin scale.
History of the Kelvin scale
British scientist, William Thomson (aka Lord Kelvin), developed the Kelvin scale. Through his studies on the relationship between the volume and temperature of gases, he theorized the volume of gas would become zero at a temperature of -273° C.
The idea of infinite cold was born, which was a theoretical point where molecules don't move. Lord Kelvin made it his life's work to calculate the temperature of this infinite cold. His first scientific paper on the topic called for the necessity of a temperature scale not relative to the properties of the material substance. On such a scale, all degrees would have the same value regardless of if the material was heated or cooled.
Lord Kelvin reshaped his initial concepts and theories over the years. Eventually, he partnered with another brilliant scientist by the name of James Prescott Joule. Their combined methods refined Lord Kelvin's initial theory, and together they came up with the present-day Kelvin scale. At absolute zero, particles stop moving. Their work also led to the discovery of the triple point, where water exists in three phases at once! Solid, liquid, and gas all at a temperature of 273 K. This temperature is known as thermal equilibrium.
Do we actually use the Kelvin scale?
Yes! Kelvin is a part of the International System of Units (SI), or more commonly known as the metric system. It is popular with scientists and researchers because there are no negative numbers. Many of the equations used within the research community would not work if not for this scale. Meteorologists use the Kelvin scale in the equations and calculations behind all the weather models. Without these equations computing the data, we would not be able to make a forecast. We use the Kelvin scale in the Ideal Gas Law, when calculating virtual temperature, in the hypsometric equation, or when calculating lapse rates. Again, these concepts and equations help produce weather models!
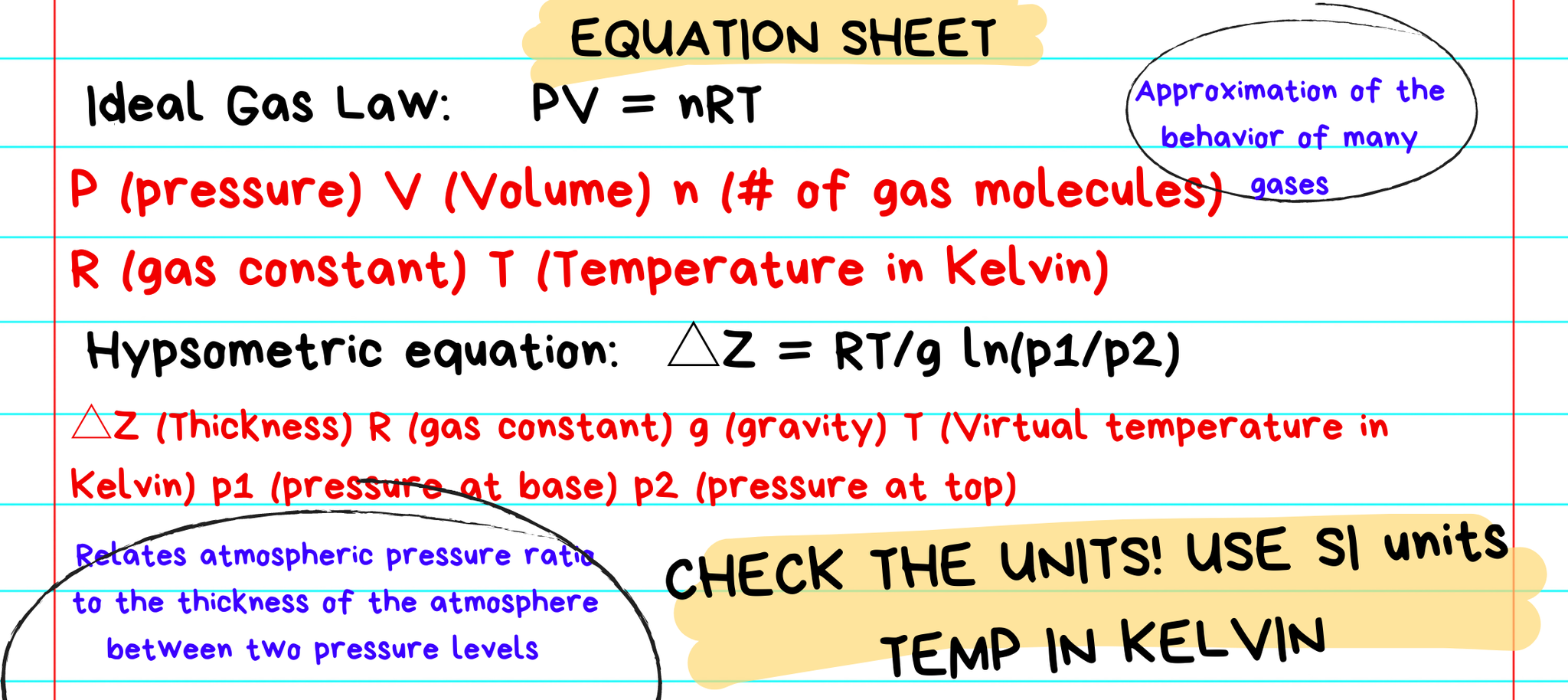
Engineers use the scale for describing the color temperatures of lights. The higher degree of Kelvin, the higher the temperature. Household lights range from 2700 K – 3000 K. Daylight has a temperature of 5000 K to 6500 K.
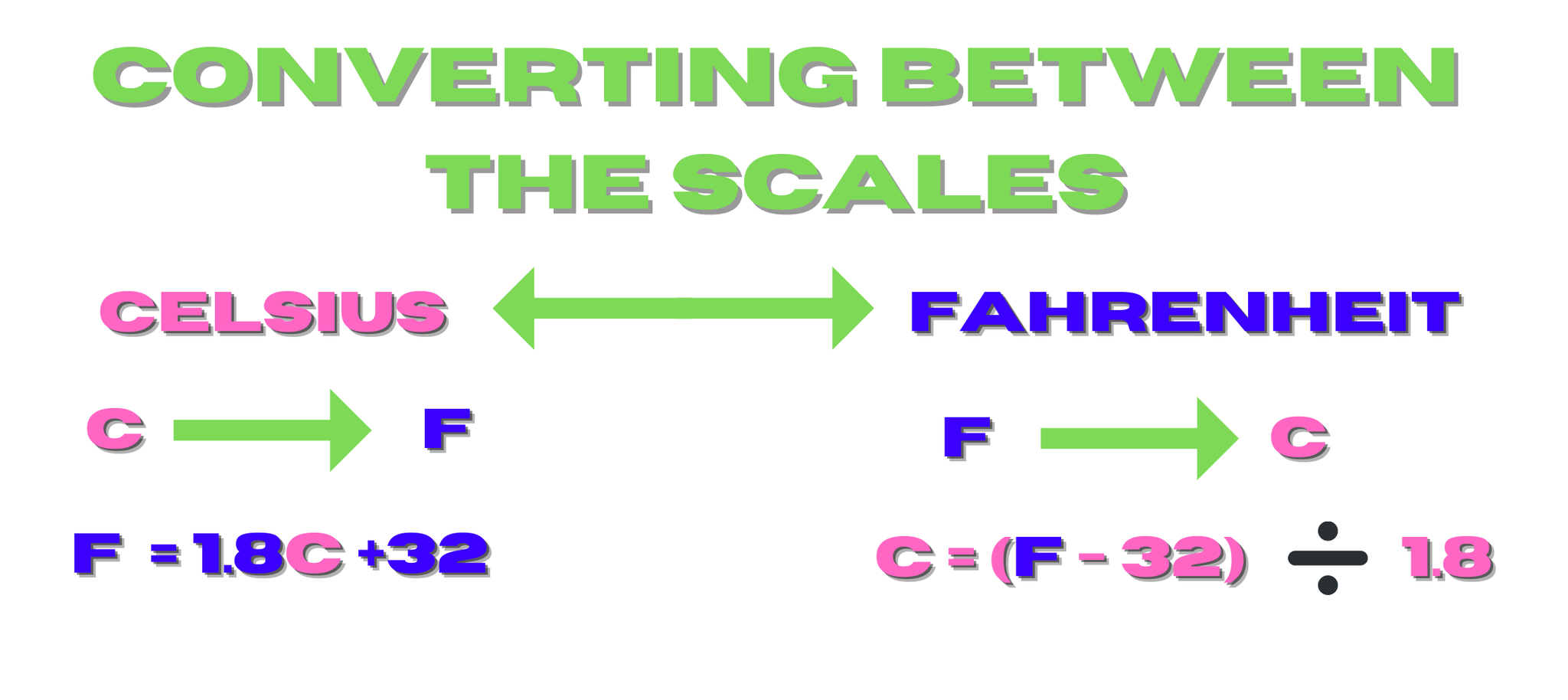
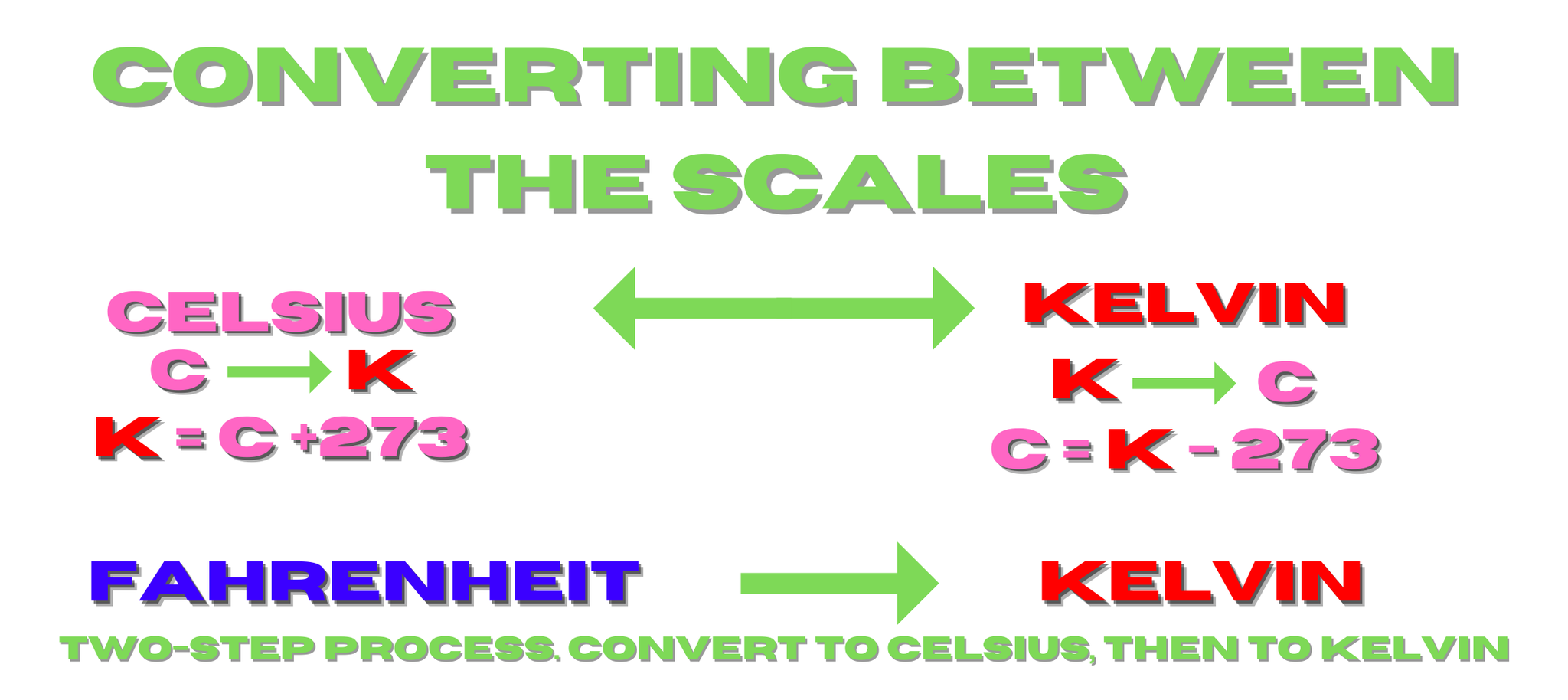
Converting between the scales
The equations below are for converting between the three temperature scales, Fahrenheit, Celsius, and Kelvin. On an important note, one degree on the Kelvin scale is the same size as a degree on the Celsius scale.
Another point, you never write degrees Kelvin, instead just use K.

0 Comments Add a Comment?